We are an affiliate
Newsatw.com is a participant in the Amazon Services LLC Associates Program, an affiliate advertising program designed to provide a means for sites to earn advertising fees by advertising and linking to Amazon.co.uk.“As an Amazon Associate, I earn from qualifying purchases.”
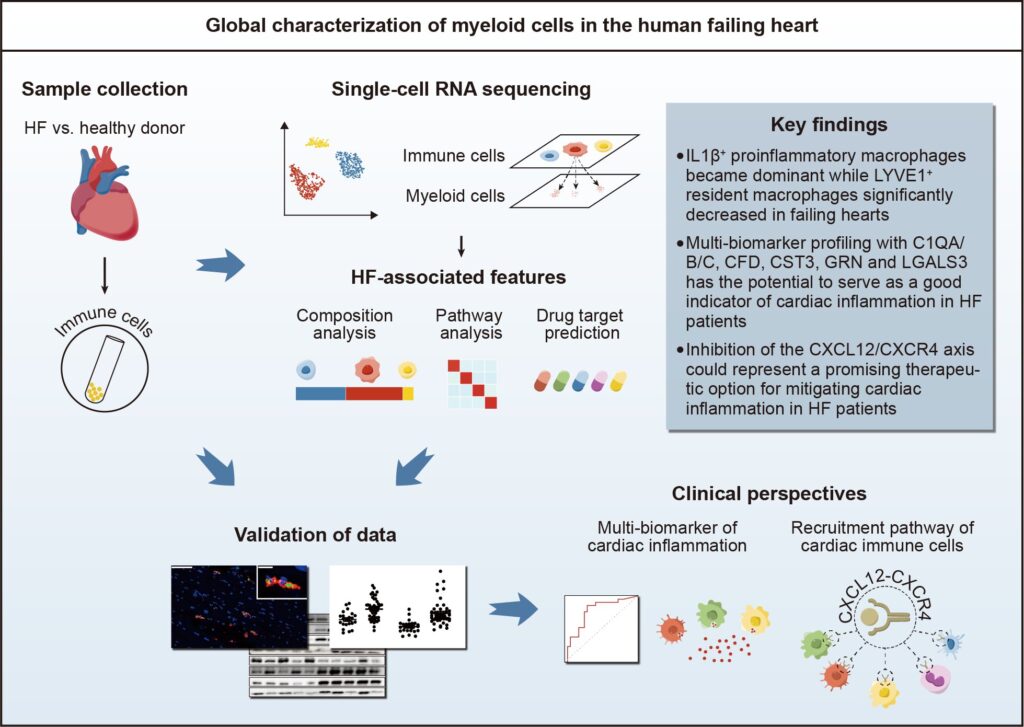
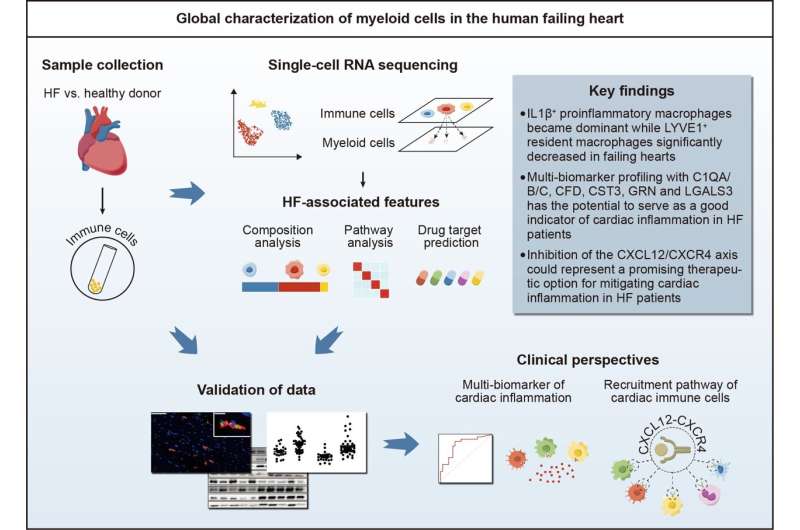
In a recent study published in the Science Bulletin, researchers comprehensively examined the composition and phenotypic features of myeloid cells in human failing hearts at a single-cell level, offering valuable insights into potential targets for monitoring and treating cardiac inflammation in heart failure.
This study was led by Prof. Xiang Cheng (Department of Cardiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology). In this study, researchers employed single-cell RNA sequencing on cardiac immune cells from heart failure patients (ischemic cardiomyopathy and dilated cardiomyopathy) undergoing heart transplantation and from healthy donors.
Transcriptomic characteristics associated with heart failure were identified. In addition to the bioinformatics analysis, experimental validations were conducted to further substantiate the reliability of the findings.
Macrophages emerged as the most abundant immune cells in normal and failing hearts, with differential expression of genes related to tissue residency, inflammation and fibrosis. In normal human hearts, Mac-LYVE1 expressing resident macrophage-associated genes dominated the macrophage population, but their proportion decreased in failing hearts.
In contrast, Mac-IL1β, which surged significantly in failing hearts, exhibited high expression of genes linked to proinflammatory and antigen-presenting function.
Heart samples from patients with heart failure are typically unavailable for clinical assessment, posing a challenge to directly evaluating cardiac inflammation. Inflammatory biomarkers signaling cardiac inflammation have emerged as a viable alternative approach.
In this study, a multi-biomarker profiling utilizing complement C1q, complement factor D, cystatin C, progranulin, and galectin-3 demonstrated promising potential as reliable indicators of cardiac inflammation in heart failure.
Chemokine receptor analysis plays a crucial role in unraveling the mechanisms of immune cell migration, which is of great significance for immunotherapies. A key finding of this research is the upregulation of CXCR4 in myeloid cell clusters within failing hearts compared to those in normal hearts. The CXCL12/CXCR4 axis potentially orchestrates the recruitment of myeloid cells to failing hearts, highlighting it as a promising therapeutic target for addressing heart failure.
Additionally, drug prediction analysis revealed drugs that potentially target myeloid cell subpopulations associated with heart failure, some of which have demonstrated potential efficacy in impeding the progression of heart failure.
More information:
Si Zhang et al, Global characterization of myeloid cells in the human failing heart, Science Bulletin (2024). DOI: 10.1016/j.scib.2024.03.042
Citation:
Unveiling immune features of heart failure paves the way for targeted therapies (2024, April 24)
retrieved 24 April 2024
from https://medicalxpress.com/news/2024-04-unveiling-immune-features-heart-failure.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
Best Electronic Deals.
Discover the Best Electronic Deals on Amazon Today. Grab Hot Discounts on Top Tech! Don't Miss Out! 🔥 Save Big Now!







![[Package content] 2 mosquito head nets and 2 storage bags, the color is black, and the size is 42*46cm. [Material] The head protecting net is made of net yarn. It is so lightweight to portable to store, very convenient to carry. Machine washable. [Fe...](https://m.media-amazon.com/images/I/41wNV+jWIhS._SL160_.jpg)












